











Reference the Sterling Mine GANJ_Calender_08.
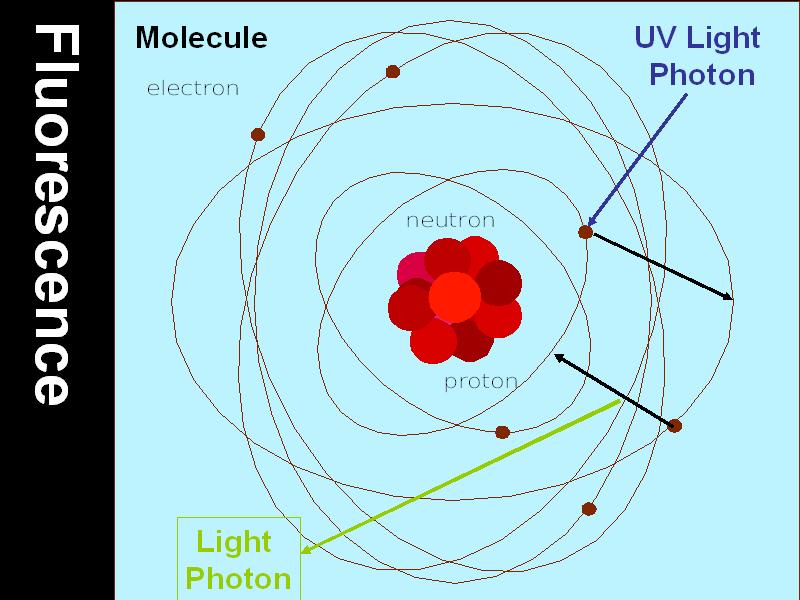
The fluorescence of Franklin minerals is most spectacular under short-wave ultraviolet light,
which has an energy of about 5 electron volts.
The ultraviolet light kicks electrons from a ground state to a high energy state.
The high energy state is unstable, and the electrons return to the ground state.
In the fluorescent minerals, energy is given off as light when electrons lose energy in a single, discrete step.
In calcite from Franklin, the electrons lose 1.8 electron volts of energy in one step, and red light, which has an energy of 1.8 electron volts is given off.
For willemite, there is an energy drop of 2.3 electron volts, and green light with an energy of 2.3 electron volts is given off.
The Franklin/Ogdensburg area all together has yielded 89 fluorescent species so far, more than found anywhere else on earth in one place.
Fictional story:
In Star Trek episode 35, the starship Enterprise goes to the planet Ogden to buy fluorescent minerals to power their photon torpedo (get it haha)